What Are the Most Common Treatment Solutions?
Cr(VI) can be removed from water in a few well-established ways. Since the initial MCL, the understanding of treatment technologies has significantly improved the efficiency and reliability of the different approaches. Current technologies include:
Non-Regenerable Strong-Base Anion Exchange ⌄
SBA-IX is a commonly implemented treatment technology for nitrate, arsenic, perchlorate, and other anionic contaminants in groundwater. SBA-IX treatment for Cr(VI) is well understood, with more than a dozen systems operating across California either in ‘non-regenerable’ (SBA-IX-NR) or ‘regenerable’ (SBA-IX-R) modes of operation.
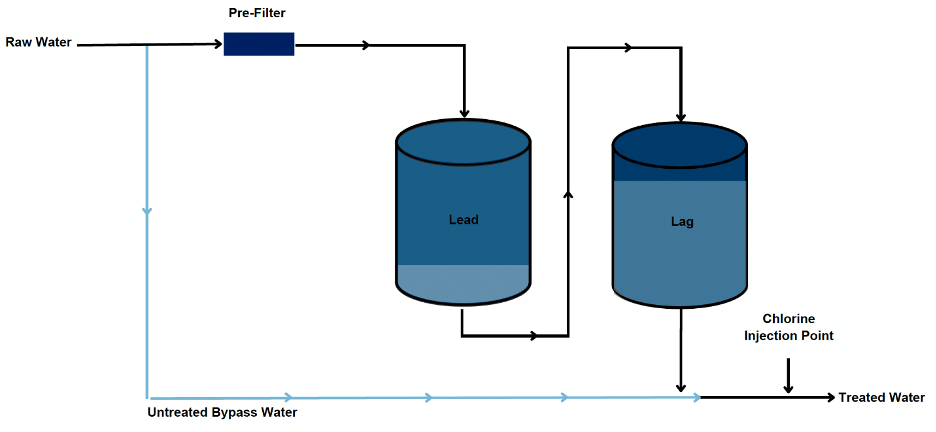
The removal of Cr(VI) in SBA-IX-NR systems is achieved similarly to WBA-IX treatment; however, SBA-IX does not require pH suppression to efficiently maintain Cr(VI) removal. As with WBA-IX, a Cr(VI) selective resin is loaded into pressure vessels and typically operated in a lead-lag configuration following prefiltration to prevent hydraulic fouling. When the resin’s capacity for Cr(VI) is exhausted in the lead vessel, the resin is replaced with fresh resin, and the lag vessel is placed into the lead position. The exhausted resin is then characterized to allow for its disposal at the appropriate waste facility. To Corona’s knowledge, most exhausted SBA-IX-NR resins have been designated as non-hazardous and have not required specific disposal requirements. However, the character of the exhausted resin is water quality specific and will need to be evaluated for each implementation.
For Cr(VI) removal, SBA-IX resin performance is largely governed by the presence of competing anions, namely sulfate and to a lesser extent nitrate, in the raw water. Resin performance, along with well utilization, directly impacts long-term O&M expenditures. This treatment approach may be generally cost competitive in cases where the SBA-IX resin can achieve sufficiently high throughput prior to Cr(VI) exhaustion, where relatively low Cr(VI) removal is required to achieve compliance, or where well utilization is low (i.e. for peaking wells). A simplified schematic of a typical SBA-IX-NR treatment process is presented, showing the pre-filter assembly required to remove particles ahead of the pressure vessels, and optional raw water bypass blending.
Regenerable Strong-Base Anion Exchange ⌄
SBA-IX is a commonly implemented treatment technology for nitrate, arsenic, perchlorate, and other anionic contaminants in groundwater. SBA-IX treatment for Cr(VI) is well understood, with more than a dozen systems operating across California either in ‘non-regenerable’ (SBA-IX-NR) or ‘regenerable’ (SBA-IX-R) modes of operation.
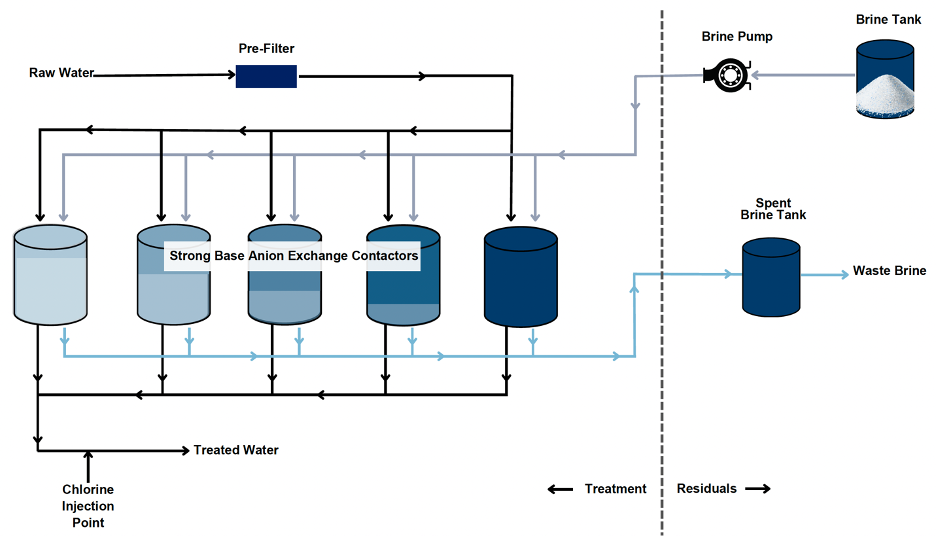
For SBA-IX-R technology, the removal of Cr(VI) is similar to WBA-IX and its SBA-IX-NR counterpart, where chloride and other anions are released into the treated water in exchange for Cr(VI) anions. By operating in a staggered parallel sequence, the system can efficiently achieve target water quality conditions by blending the finished water effluents. When a single pressure vessel reaches the target breakthrough, the resin is regenerated on-site with a brine solution, typically NaCl at a concentration of 10% to 12% by weight, to restore the resin’s capacity to remove Cr(VI). A simplified schematic of a typical SBA-IX-R treatment process is presented in Figure 10, showing both the treatment and residual streams for on-site regeneration. Resin regeneration typically requires on-site sodium chloride and fresh brine storage, along with spent regenerant brine storage. The disposal of this regenerant brine is often the most significant challenge for SBA-IX-R treatment implementation due to the brine’s elevated Cr(VI) and chloride concentrations, typically rendering it a hazardous waste. Depending on the selected regeneration process, regenerant brine may contain Cr(VI) concentrations more than 1 g/L. At these Cr(VI) concentrations, the waste brine will likely exceed the 5 mg/L Cr(VI) threshold for hazardous waste classification, requiring additional disposal considerations. Other contaminants removed during SBA-IX-R treatment (e.g. arsenic, vanadium, selenium and uranium) can also accumulate in the waste brine at hazardous concentrations. Most existing Cr(VI) SBA-IX-R systems, rely on spent regenerant brine transportation to a licensed Treatment, Storage, and Disposal Facility (TSDF) that recovers the chromium and other materials in the waste brine for beneficial reuse. Alternatively, the regenerant brine can be chemically treated on-site to render it non-hazardous. Solids produced during this brine treatment are then disposed at a non-RCRA (Resource Conservation and Recovery Act) California hazardous waste facility or Low-Level Radioactive Waste (LLRW) facility depending on the waste characterization. This approach adds additional complexity to the process but could result in operational cost savings.Weak-Base Anion Exchange (WBA) ⌄
WBA-IX relies on pH adjustment of the influent water to as low as 5.5 SU to effectively remove Cr(VI) to 2 µg/L or less. WBA-IX systems generally consist of pressure vessels loaded with Cr(VI) specific WBA-IX resin, configured in a lead-lag orientation. WBA-IX resins are prone to fouling and require a pre-filter assembly to remove particles ahead of the pressure vessels. Chemical feed and storage facilities are required to store and dose chemicals (i.e., acid and base) for pH adjustment. Additionally, if carbon dioxide is selected for pH depression, an aeration system is also needed. When the resin capacity for Cr(VI) of the lead vessel is exhausted, the resin is replaced, and the lag vessel is placed into the lead position through a manual valve manifold. This approach to operation of lead-lag vessels provides confidence in the finished water quality, as the lag vessel serves as a polishing step while improving the overall resin use rate. A simplified schematic of a typical WBA-IX treatment process is presented, where the “waste backwash water” flow path is only used to rinse fresh resin upon initial treatment start-up or following a resin replacement.
Following pH depression to 5.5 SU and passing through the lead-lag filter vessels, post-treatment adjustment to raise the pH is needed to avoid adverse impacts to the distribution system (e.g., corrosion, scaling, taste and odor issues, etc.). The magnitude of the chemical addition is dependent on the source water alkalinity, with operational costs increasing with increasing alkalinity. Hydrochloric acid (HCl) has generally been used for WBA-IX applications to eliminate the potential resin interference from sulfate ions in sulfuric acid (H2SO4), and sodium hydroxide (NaOH) is typically used for post-treatment adjustment. Carbonic acid (H2CO3) can also be used through CO2 addition, in which case, aeration is utilized for post-treatment pH adjustment. It is important to note that mineral based acids (e.g., HCl or H2SO4) and NaOH will increase the TDS entering the distribution system proportionally to the raw water alkalinity. Previous research completed as part of Water Research Foundation (WRF) project 4450, Impact of Water Quality on Hexavalent Chromium Removal Efficiency and Cost demonstrated the ability of WBA- IX resins to reliably remove Cr(VI) at concentrations ranging from of 10 to 35 µg/L for a minimum of 150,000 bed volumes (BVs). Note that one BV is equivalent to the volume of resin being used in the treatment process; therefore, at 150,0000 bed volumes, 1-gallon of WBA-IX resin would be capable of producing 150,000 gallons of treated water with a Cr(VI) concentration less than 10 µg/L. The WBA-IX resin is operated as a single use product, with the exhausted resin requiring special disposal considerations. Cr(VI) specific WBA-IX resins would be classified as a hazardous waste in California after its treatment capacity for Cr(VI) is consumed. In addition to Cr(VI), WBA-IX can also remove inorganic elements such as copper, vanadium, and uranium. In particular, source water uranium is a concern for this technology, as the Cr(VI) exhausted resin could be classified as non-RCRA (Resource Conservation and Recovery Act) California Hazardous Waste and potentially Low-Level Radioactive Waste (LLRW) due to uranium accumulation.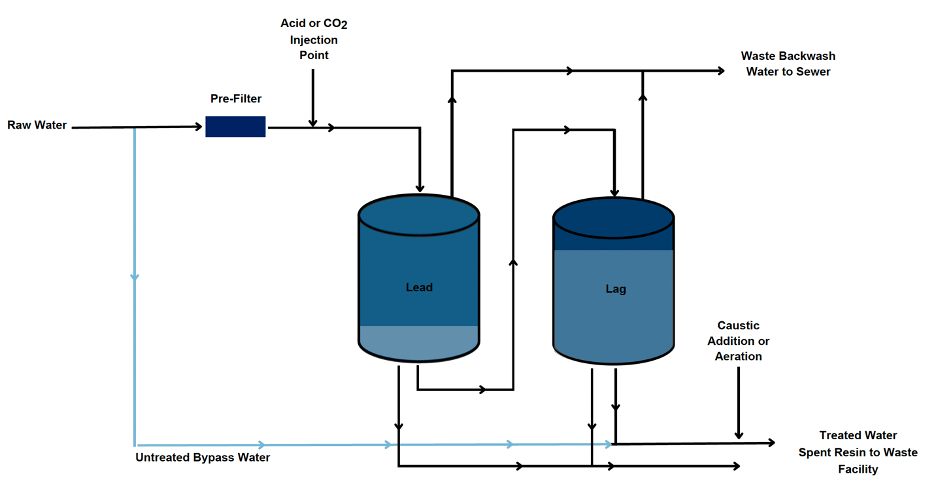
Reduction–Coagulation–Filtration (RCF) ⌄
The RCF treatment process removes chromium by first reducing Cr(VI) to the less soluble trivalent chromium [Cr(III)] species, as chromium hydroxide [Cr(OH)3], with the addition of a chemical reductant, typically ferrous iron [Fe(II)] which is provided as a bulk liquid chemical as either ferrous chloride (FeCl2) or ferrous sulfate (FeSO4). Both reductants are equally effective in this approach at the same dose as iron (i.e., mg/L as Fe). The reduction of Cr(VI) as chromate (CrO42-) to Cr(III) as chromium hydroxide [Cr(OH)3(s)] is assumed to proceed according to the following equation:
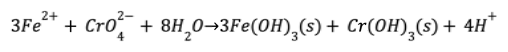
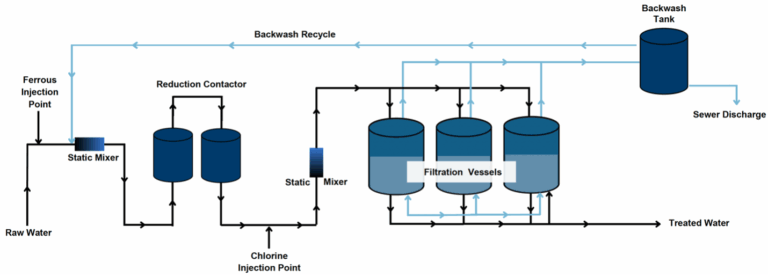
where Fe(OH)3(s) is ferric hydroxide, a typical form of ferric iron. Since Fe(II) is typically dosed in excess of the stoichiometric ratio, chlorine is injected following the requisite reduction time to facilitate oxidation of residual Fe(II) to Fe(III) and form Fe(OH)3 particles. It is assumed that Cr(III) is adsorbed to Fe(OH)3 particles, which are subsequently removed through filtration. Periodically, the filters are backwashed to remove accumulated particles and restore hydraulic capacity. A simplified schematic of the RCF treatment process is presented. While not included in the process diagram, limited data suggests that a polymer filter aid may increase filter run time and decrease total volume of backwash water and should be considered when testing this technology. The RCF process has long been used in industrial Cr(VI) treatment; however, full-scale drinking water experience has been limited to a single potable water system operating in Las Lomas, CA and a demonstration system in Glendale, CA. During compliance efforts with the initial Cr(VI) ruling, a 30-minute reaction time was thought to be needed to effectively reduce Cr(VI) with Fe(II), followed by aeration to oxidize the residual Fe(II) to form Fe(OH)3 particles for removal through filtration. Research conducted by Corona over the last decade shows that this reaction time could be as little as 1 to 2 minutes and that the equivalent Cr(VI) reduction could be achieved with longer reaction times at a given Fe(II) dose. Corona’s research also found that following the requisite reaction time, chlorine could be injected in lieu of aeration to aid in the oxidation of any residual Fe(II) to form Fe(OH)3 particles. These treatment advances greatly simplified the treatment process and reduced the overall footprint requirements. Furthermore, depending on the water quality, pilot testing has shown that reduction times as low as 1 minute and ferrous doses as low as 1 mg/L can be effective. Backwash residuals can be discharged directly to sewer if water quality meets local discharge limits and sewer capacity is able to accommodate the additional flow. Residuals can also be directed to a storage tank to facilitate solids settling and backwash recycling. Backwash recycling improves overall water efficiency of the treatment process; settled backwash solids would be periodically removed from the bottom of the tank and discharged to sewer. If settled backwash solids cannot be discharged to sewer then solids must be managed on-site by 1) allowing backwash solids to accumulate in a storage tank until sufficient volume has been generated for off-site disposal, 2) directing the settled solids to a dewatering and drying container, or 3) mechanically dewatering the solids with a belt filter press or centrifuge. These approaches will concentrate Cr(VI) and other co-precipitated metals (i.e., arsenic, manganese) in solids and decrease the water content of the material for disposal, both of which can impact hazard classification and ultimate disposal. Regardless of disposal approach, waste will require characterization prior to selecting the appropriate disposal facility.
Reverse Osmosis (RO) ⌄
RO is a widely accepted high-pressure membrane technology capable of removing a variety of naturally occurring and anthropogenic compounds from drinking water. The core mechanism in RO treat is highly pressurized water traveling through a membrane, separating ions and cations from the water through electrostatic repulsion and size exclusion. The resulting waste stream is a brine with high concentrations of dissolved constituents, which did not pass through the membrane and requiring separate processing for disposal. Utilities in inland areas may face challenges with disposal of this highly concentrated brine. Additionally, small to medium sized utilities may find the high electrical costs associated with RO treatment to be restrictive. Due to these challenges, RO it is not commonly considered for Cr(VI) removal.
What about Stannous Chloride without filtration?
In 2014, our team began work evaluating the efficacy of stannous chloride addition without filtration as a viable treatment alternative for Cr(VI) MCL compliance. With the 2017 invalidation of the MCL, this work went on hiatus, with work resuming in 2023. Unfortunately, due to particulate tin and chromium formation, we are not currently recommending that drinking water utilities pursue this as a potential treatment for Cr(VI) impacts to drinking water.
Our work on this subject is summarized in an AWWA Water Science article published in 2024.
